Ketamine Science
- Home
- Ketamine Science
Understanding the Science of Ketamine and its Influences on the Brain
The Role of Ketamine in Creating New Synapses
- Ketamine acts on the NDMA receptor to release a flood of glutamate, stimulating growth factors like brain-derived neurotrophic factor (BDNF) and creating new connections or synapses in your mind.
- This remarkable medication can relieve pain, reduce stress and enhance memory formation. It accomplishes this by restoring connections within your brain’s previously damaged networks due to chronic illness or trauma.
- Triggering the release of growth factors like BDNF (brain-derived neurotrophic factor) helps make new synapses (connections)and thought patterns that lead to new perspectives and insights on life.
- In some patients, ketamine treatments offer relief from anxiety and depression without side effects or recovery time after treatment because it goes straight to the lobes of your brain, where feelings are processed while not affecting other parts like muscles do with medication which can make you feel drowsy during the recovery period.
- Low levels of BDNF have been linked to depression, and ketamine has been shown to increase BDNF levels in the brain.
- Some other potential mechanisms for ketamine’s antidepressant effect include its action on parvalbumin interneurons (which act like a brake on brain activity) and its binding to various neuroreceptors, including dopamine, noradrenaline, nicotinic, and opioid receptors.

What is the role of Brain-Derived Neurotrophic Factor (BDNF)?
- BDNF is a protein involved in the growth and survival of neurons in the brain.
- BDNF is critical in supporting the survival and growth of healthy neurons, which helps memory and learning.
- Its effects boost cognition and play a role in modulating the brain’s neurotransmitters to promote neural plasticity.
- Exercising can increase levels of BDNF mRNA
- BDNF stimulates neurogenesis (growth of new neurons from neural stem cells)
- It enhances neurons’ survival when exposed to injuries, oxidative stress, axotomy, and hypoxic-ischemic injury.
- The influence of BDNF is expressed in the hippocampus. It has been identified in other brain areas, such as the olfactory bulb, cortex, basal forebrain, mesencephalon, hypothalamus, brainstem, and spinal cord.
- BDNF helps regulate activity-dependent synaptic plasticity.
- BDNF helps synapses adapt in response to experiences, a process named synaptic plasticity.
- The increase in BDNF expression is associated with an improvement in depression-like behavior.
- Ketamine also upregulates neuronal production and release of BDNF (brain-derived neurotrophic factor), stimulates a central cell pathway called mTOR (mammalian target of rapamycin), which regulates many processes involved in cell growth, including synthesizing proteins needed for long-term memory and reverses synaptic damage that occurs in key areas of the brain associated with emotional regulation when the brain is subjected to chronic stress.
What Is Glutamate?
- Glutamate is the most abundant excitatory neurotransmitter (chemical messenger) in the central nervous system (CNS) and brain. It plays a crucial role in neuronal communication and synaptic plasticity.
- Our brains need the proper balance of glutamate to perform at their best. Too little or too much can have serious consequences. Maintaining this delicate equilibrium for our cognitive functioning is important.
- It is recycled and made by glial cells and converted to glutamine when delivered back to the nerve cell terminal area and then used as an amino acid building block.
- It helps create GABA, which aids with relaxation processes such as sleep regulation, muscle function control and managing of anxiety levels.
- Glutamate is also stored in our muscles, where we can use this neurotransmitter for protein.
- Glutamate is also a popular taste enhancer found in many favorite snacks in the form of monosodium glutamate (MSG).
- Research into ketamine’s full pharmacological action reveals that it increases the release of glutamate from the presynaptic neuron into the synaptic cleft, preferentially blocking glutamate at NMDA receptors on postsynaptic cells.
Excessive Glutamate in the Brain is Connected to:
- Amyotrophic lateral sclerosis (ALS/Lou Gehrig’s disease)
- Multiple sclerosis
- Alzheimer’s
- Parkinson’s
- Huntington’s
- Stroke
- Fibromyalgia
- Chronic fatigue syndrome.
Some researchers think it may help prevent and help manage some diseases like diabetes
Inadequate Glutamate levels MAY Result In:
- Problems concentrating
- Being mentally exhausted
- Insomnia (unable to sleep)
- Lethargy (lack of energy)

What Is Glutamate Neurotransmission?
- Glutamate neurotransmission refers to the release, binding, and removal of glutamate in the synaptic cleft between neurons, which is necessary to transfer information from one neuron to another.
- Glutamate is released into the synaptic cleft and binds to receptors on the postsynaptic neuron, triggering an electrical signal in the postsynaptic neuron. The glutamate is then taken back into the presynaptic neuron by glutamate transporters or metabolized by enzymes, which terminates the neurotransmission.
- The balance between excitatory and inhibitory neurotransmission, which is regulated by the activity of different neurotransmitter systems, is crucial for normal brain function and regulation of mood, memory, and other cognitive processes.
- Alterations in glutamate neurotransmission have been implicated in several neurological and psychiatric disorders, including depression, anxiety, and schizophrenia, among others. Some antidepressant and antipsychotic medications target the glutamate system to improve symptoms.
How Glutamate Neurotransmission Works?
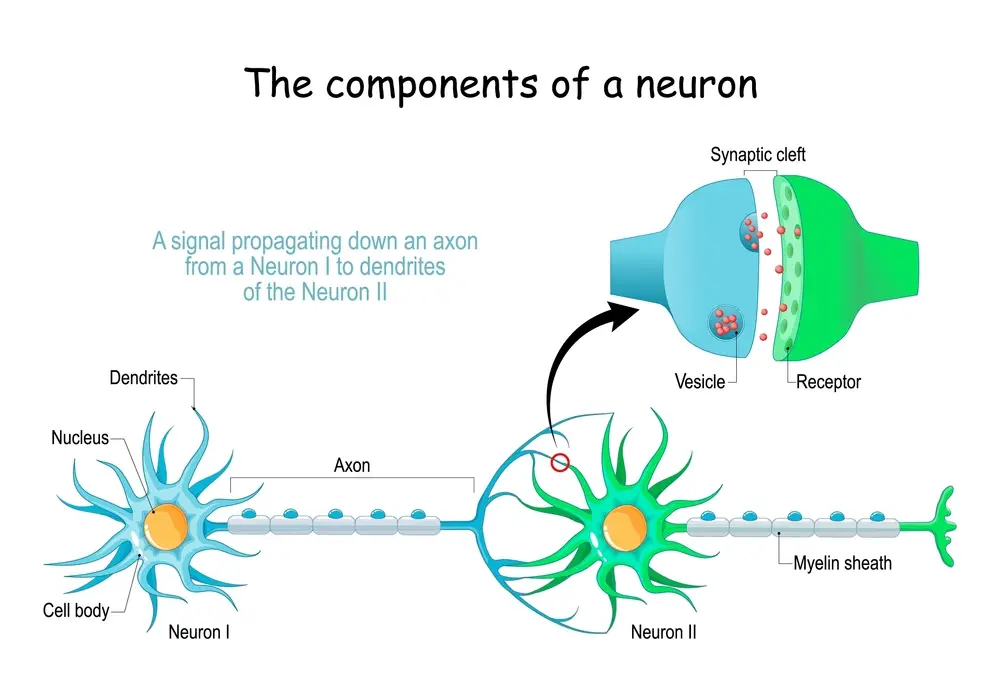
Release
When an action potential reaches the terminal of a presynaptic neuron, voltage-gated calcium channels open, allowing calcium ions to enter the terminal. This increase in intracellular calcium triggers the release of glutamate into the synaptic cleft.
Binding
Glutamate diffuses across the synaptic cleft and binds to specific receptors on the postsynaptic neuron, including NMDA (N-methyl-D-aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), and kainate receptors. The binding of glutamate to these receptors triggers changes in the postsynaptic membrane potential, leading to the initiation of an electrical signal in the postsynaptic neuron.
Removal
After neurotransmission, the glutamate is removed from the synaptic cleft. This is accomplished by reuptake into the presynaptic neuron by glutamate transporters or by metabolic degradation by enzymes such as glutamate dehydrogenase.
What are NMDA receptors? What do they do and how does Ketamine affect them?
- NMDA receptors (N-methyl-D-aspartate receptors) are a type of glutamate receptor in the brain.
- NMDA receptors are involved in several important brain functions, including learning and memory, neuronal excitability, and synaptic plasticity.
- They are also involved in regulating mood and the modulation of pain perception.
- Ketamine is a non-competitive antagonist at the NMDA receptor, meaning it blocks the binding of the neurotransmitter glutamate to the receptor.

- By blocking the action of glutamate at NMDA receptors, ketamine can produce a range of effects on brain function and behavior.
- In addition to its anesthetic properties, ketamine has been shown to have rapid and robust effects treating depression, especially in patients who have not responded to other treatments. The exact mechanisms by which ketamine produces its antidepressant effects are not fully understood. They are thought to involve changes in the activity of specific brain circuits involved in mood regulation and changes in the levels of neurotransmitters such as glutamate, dopamine, and norepinephrine.
- While the effects of ketamine on NMDA receptors may be responsible for some of its therapeutic benefits, they can also result in side effects such as hallucinations and dissociation, as well as potential risks to cognitive and cardiovascular function, especially with long-term use. For these reasons, much more research is necessary to validate the safety and efficacy of ketamine in various psychiatric and neurological conditions.
- Ketamine acts as an NMDAR inhibitor (N-Methyl-D-aspartate receptor)
Is a Decrease in BDNF Correlated to Neurodegenerative Diseases?
There is an association between reduced levels of BDNF and neurodegenerative diseases with neuronal loss in people with multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and Huntington’s Disease.

